COVID-19 Vaccination Update
As we anticipate the arrival of the first COVID-19 vaccines in our community, we at AIM Health are working to ensure you are continually updated. This message is the first update of several we will provide as we learn more details and watch the vaccination process unfold. In this update, we address:- What types of vaccines are coming.
- Vaccine effectiveness.
- How the two vaccines work.
Additionally, we’ve outlined the details on when and how you can get a vaccine in another blog post. We hope this is helpful information and helps clarify any initial questions you may have.
What Types of Vaccines Are Coming?
Currently, there are two main vaccines at the forefront of development, one from Pfizer and one from Moderna, with others in the pipeline. Neither the Pfizer nor Moderna vaccines have FDA approval at this time, but both have applied for emergency use authorization (EUA). It appears that Pfizer’s vaccine will be voted on for EUA 12/10/20, and Moderna’s 12/17/20, with initial distribution soon after, if approved.Are They Safe and Effective?
 The vaccine trials were spilt into participants who received the vaccine and participants who received a placebo.
Pfizer had 44,000 people in its vaccine trial. 94 participants became ill with COVID-19. The scientists looked at how many of these 94 participants received the vaccine and how many received a placebo and determined that the vaccine is over 90% effective.
Moderna recruited 30,000 volunteers across the United States to participate in its vaccine trial. Of the 30,000 participants, 196 people became ill, 30 became severely ill, all of whom had received a placebo. This suggests that the vaccine can block the virus, but can also help people who do get sick from having more severe symptoms. Both safety and efficacy will be evaluated during the EUA hearings based on studies done over the last 10 months. Participants are being carefully tracked for side effects after receiving the vaccines. Both vaccines are expected to reduce the risk of developing symptomatic COVID-19 infection by about 95%.
The vaccine trials were spilt into participants who received the vaccine and participants who received a placebo.
Pfizer had 44,000 people in its vaccine trial. 94 participants became ill with COVID-19. The scientists looked at how many of these 94 participants received the vaccine and how many received a placebo and determined that the vaccine is over 90% effective.
Moderna recruited 30,000 volunteers across the United States to participate in its vaccine trial. Of the 30,000 participants, 196 people became ill, 30 became severely ill, all of whom had received a placebo. This suggests that the vaccine can block the virus, but can also help people who do get sick from having more severe symptoms. Both safety and efficacy will be evaluated during the EUA hearings based on studies done over the last 10 months. Participants are being carefully tracked for side effects after receiving the vaccines. Both vaccines are expected to reduce the risk of developing symptomatic COVID-19 infection by about 95%.
How Do They Work?
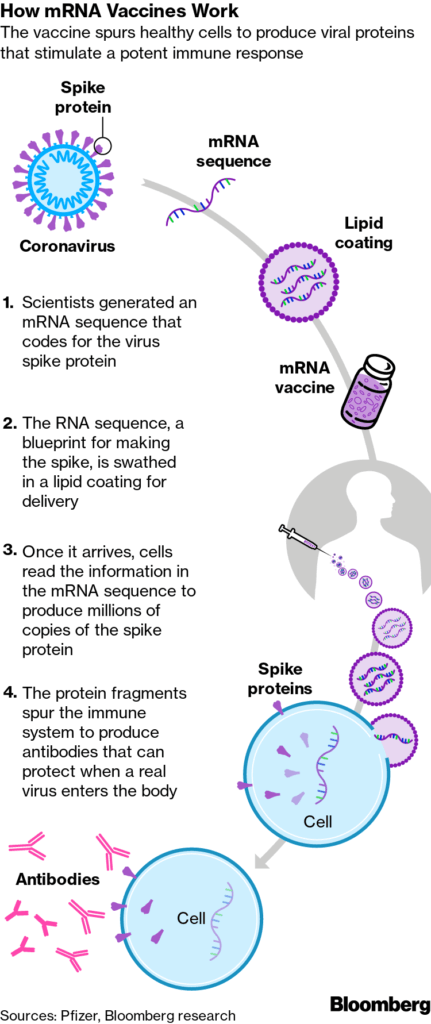 Both vaccines use messenger RNA technology. These are the first vaccinations to use this method. Traditional vaccines use a weak or dead piece of a virus or proteins generated in a lab to elicit an immune response. Both the Pfizer and Moderna vaccines use a piece of the virus’s genetic code (mRNA) to elicit the response as illustrated in the graphic.
The Pfizer vaccine will require two injections given 21 days apart. It needs to be kept at -94°F complicating transportation and distribution of the vaccine. The Moderna vaccine requires two injections 4 weeks apart and needs to be transported at -4°F. At this time the length of protection from either vaccine is not known.
Both vaccines use messenger RNA technology. These are the first vaccinations to use this method. Traditional vaccines use a weak or dead piece of a virus or proteins generated in a lab to elicit an immune response. Both the Pfizer and Moderna vaccines use a piece of the virus’s genetic code (mRNA) to elicit the response as illustrated in the graphic.
The Pfizer vaccine will require two injections given 21 days apart. It needs to be kept at -94°F complicating transportation and distribution of the vaccine. The Moderna vaccine requires two injections 4 weeks apart and needs to be transported at -4°F. At this time the length of protection from either vaccine is not known.